What percent of the SCN − present initially have been converted to FeSCN2 at equilibrium?
Lab 11 - Spectroscopic Determination of an Equilibrium Constant
Goal and Overview
The reaction of iron (Iii) with thiocyanate to yield the colored production, iron (III) thiocyanate, can exist described by the following equilibrium expression.
( one )
Fethree+ + SCN− 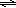 FeSCNii+
FeSCNii+
You will written report this equilibrium using the Spec 20 UV-visible spectrometer. The wavelength of lite absorbed most strongly by the product will exist determined from the spectral contour of FeSCNii+. A Beer's Law plot volition be made for a serial of FeSCN2+ solutions of known concentration. Then, the concentrations of FeSCN2+ will be measured spectroscopically for a ready of solutions made with different initial concentrations of reactants. This data volition be used to decide K eq,
Objectives and Scientific discipline Skills
-
•
Perform volumetric dilutions and calculate resulting molarities. -
•
Empathize and explain assimilation spectroscopy and the mathematical relationships betwixt percentage transmittance, absorbance, concentration, path length, and extinction coefficient. -
•
Apply linear fitting methods to find relationships between dependent and independent variables, such as percent transmittance (absorbance) and concentration. -
•
Explain and apply Beer's Constabulary; draw the assumptions and limitations imposed by the nature of the equilibrium on the calculation of FeSCN2+ associated with the absorption data. -
•
Use absorption information to qualitatively and quantitatively analyze the concentration of FeSCN2+ in solution. -
•
Identify and discuss factors or effects that may contribute to the uncertainties in values or assessments made from experimental data. -
•
Analyze, quantify, and discuss the uncertainty in results when assumptions are used.
Suggested review and external reading
-
•
Reference information on spectroscopy (see Lab nine) and dilutions; relevant textbook information on spectroscopy and equilibrium
Background
This experiment investigates the equilibrium established by the reaction of the iron (Iii), Feiii+, and the thiocyanate, SCN–, ions. See Eq. 1 Fe3+ + SCN− 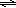 FeSCN2+
FeSCN2+
One goal in this experiment is to mensurate the value of Grand, using the spectrometer to quantitatively analyze the concentration of FeSCNtwo+ ion. Absorption spectroscopy and Beer'due south Police were discussed in detail and were used in the Allura Scarlet Lab. This same method will be utilized to determine [FeSCN2+], the colored production.
( 4 )
T = = 10− ε bc = 10− A
Then, yous will use your understanding of equilibrium processes to deduce the equilibrium concentrations of the reactants. Knowing all three concentrations listed above in Eq. 2 Thousand =
Procedure
Function 1: Qualitative Observations: Is the reaction exothermic or endothermic?
1
Using a 10 mL graduated cylinder, mensurate out approximately 2 mL of 2 × 10–3 M NaNO3 and put it in a exam tube.
2
Add together approximately 8 mL of 2 × 10–3 1000 NaSCN.
3
Add approximately 10 mL of two × 10–3 Chiliad Fe(NOthree)iii. Note the color of the solution.
four
Fill up a Spec 20 cuvette no more than than 2/3 full, and split the remaining solution among iii test tubes.
five
Place one tube in an ice bath and i in the hot water bath on the hot plate.
six
Subsequently about ten minutes, compare them with the solution at room temperature.
7
Talk over the implications of your observations, basing your discussion on your noesis of Le Châtelier'south principle.
Do your observations imply an exothermic or endothermic reaction?
Part 2: Spectral Profile and λ max
(most sensitive wavelength) of FeSCN2+
1
Measure transmittance (%T) to 0.ane% of the mixtures in your cuvette in the range from 370 to 560 nm. a b c
2
For the wavelength of minimum %T, calculate the absorbance, A, of the solution (A = –log T) to the appropriate number of significant figures.
three
Identify the wavelength of maximum absorbance, the experimental value of λ max.
How does your λ max
4
Set your spectrometer to λ max
Function 3: Beer'southward Police Curve for FeSCN2+: determining ε of the production at λ max
To make solutions of known concentrations of FeSCN2+, you cannot merely dissolve a salt containing FeSCNtwo+ in water because the ion will dissociate in social club to satisfy the equilibrium constant expression. If M eq [FeSCN2+]eq [SCN−]initial. Grand eq,
1
Take well-nigh 100 mL of 0.i M Fe3+ solution ( solution B ) in a small labeled beaker.
2
Make the strongest colored solution of NaSCN and Fe(NO3)3 ( solution A ). a b
three
Use only volumetric glassware, not graduated pipets or cylinders. • •
Accurately create x mL volumes of the post-obit dilutions of solution A with solution B. As each of these solutions is created, measure its %T to 0.1%. a b c d e f grand
4
Calculate absorbance for each solution: A = –log(%T/100%).
5
Make a Beer'due south Law plot of absorption versus concentration of FeSCNtwo+ (A vs [FeSCN2+]) for these 7 points. Use two meaning figures in your concentration values and iii for your absorbance values.
6
Draw the all-time-fit straight line to the points.
seven
This best-fit line mathematically has the form of Beer'due south Law: A = ε bc, with slope = ε b and y-intercept = 0; ε = molar extinction coefficient in L/mol · cm, b = pathlength in cm, and c = concentration in mol/L. Determine the gradient to the ones place.
8
Using the measured pathlength b of your cuvette (to 0.01 cm) and your slope, calculate the value of the extinction coefficient for FeSCNtwo+ at its λ max
9
Record which Spec twenty you used so you tin employ the same i for Part 4.
Summary of Desired Quantities Parts i – three.
-
•
Temperature Dependence: Is the reaction exothermic or endothermic? Explain.
-
•
Spectral Profile: What isλ max?
-
•
Beer's Police Plot: Graph of Absorbance versus [FeSCN2+] noting ε atλ max.
Part iv: Equilibrium Constant for the Formation of FeSCN2+
In this part of the experiment, you will gear up five solutions with the same initial concentration of Fe3+ ion but different initial concentrations of SCN– ion. As y'all make each solution, mensurate its percentage transmittance at λ max 1000 eq G =
1
Use the solutions provided, each of which is ii × 10–3 Yard: NaSCN, Fe(NO3)3, and NaNOiii.
two
Use volumetric pipets and a 10 mL volumetric flask to ready each of the following five solutions. • • • •
Waste material disposal: The solutions must exist put into the labeled waste material bottles in the back hood. Cipher tin can get down the sink. If you are e'er in doubtfulness, ask your TA.
3
Observe equilibrium concentrations of Fethree+, SCN–, and FeSCN2+ to two significant figures. (Run across Equations one Feiii+ + SCN− One thousand = 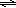 FeSCN2+
FeSCN2+
ICE tables volition help yous decide these values. Initial amounts, changes in amounts, and final equilibrium amounts are shown. These values must be in moles/50. • • V two = x mL. • • • ([FeSCN2+]eq):
[FeSCN2+] at equilibrium is determined using Beer's Constabulary; x is the corporeality of FeSCN2+ created (adamant experimentally).
( five )
x = [FeSCN2+]eq = = A / slope
To complete your ICE tables, one for each trial in Part four (concentrations should have 2 significant figures):
a b
4
Employ Eq. two Chiliad =
5
Detect the average value of K eq, the standard deviation, and the relative error (standard deviation divided by the average). Are the values of K for all trials similar? Should they be?
Assumption Check Used in Making Beer's Law Plot
Given your value of 1000, go dorsum and check the validity of the assumption you made in Function iii.
1
Rewrite Eq. 2 K = [FeSCNtwo+]eq [SCN−]eq, [FeSCN2+]eq/[SCN−]eq,
2
Summate the guess ratio of [FeSCN2+]eq/[SCN−]eq K eq K eq.
3
The supposition that essentially all of the SCN– reacted to form FeSCN2+ would mean that this ratio would need to be large. Is it? If the ratio is small, the supposition was conspicuously a bad 1 and the experiment is useless in determining the equilibrium FeSCNii+ concentration and K eq.
four
Hash out how good the assumption was and how the assumption affected the calculated values of K eq. • K eq [FeSCNii+]eq/[SCN−]eq ≈ 35. • • • [FeSCNii+]eq Thousand eq
5
Checking the assumption is only part of a thorough experimental analysis; information technology should non exist considered the main point of the lab.
Results
Complete your lab summary or write a written report (as instructed).
Abstract
Results
- Observations for Part ane
- λ max and absorbance at λ max for Part 2
- Beer's Police force plot for Office 3 including slope( ε b)
- Water ice tables, private and average
K eq
values
Sample Calculations
- Absorbance from transmittance
- Concentration calculations
-
K eq
calculation - Assumption testing
Give-and-take/Conclusions
- What you institute out and how
- Relating results to predictions/theory
- How was Role 3 dependent on Role 2?
- Validity of the supposition
- What can you conclude from this experiment
Review Questions
Source: https://www.webassign.net/labsgraceperiod/ucscgencheml1/lab_11/manual.html#:~:text=This%20means%20that%2035%20out,due%20to%20just%20the%20assumption.
0 Response to "What percent of the SCN − present initially have been converted to FeSCN2 at equilibrium?"
Post a Comment